Natural vs Lab-Grown Stem Cells: Understanding Differences, Benefits, and Therapeutic Uses
Stem cells are undifferentiated biological units capable of self-renewal and specialization, driving regenerative therapies across medicine. Comparing natural stem cells—harvested directly from bone marrow, cord blood, or adipose tissue—and lab-grown counterparts such as iPSCs and ESCs reveals critical contrasts in immune compatibility, scalability, ethical debate, cost, and clinical outcomes. This guide maps how natural sources supply autologous and allogeneic cells, how in vitro culture and reprogramming yield pluripotent lines, and how these cell types influence transplantation procedures, therapeutic applications, and future innovations. You will learn foundational sources, manufacturing methods, comparative immune responses, procedure workflows, current clinical uses, and projections for gene editing, personalized medicine, and market growth.
What Are Natural Stem Cells and Their Primary Sources?
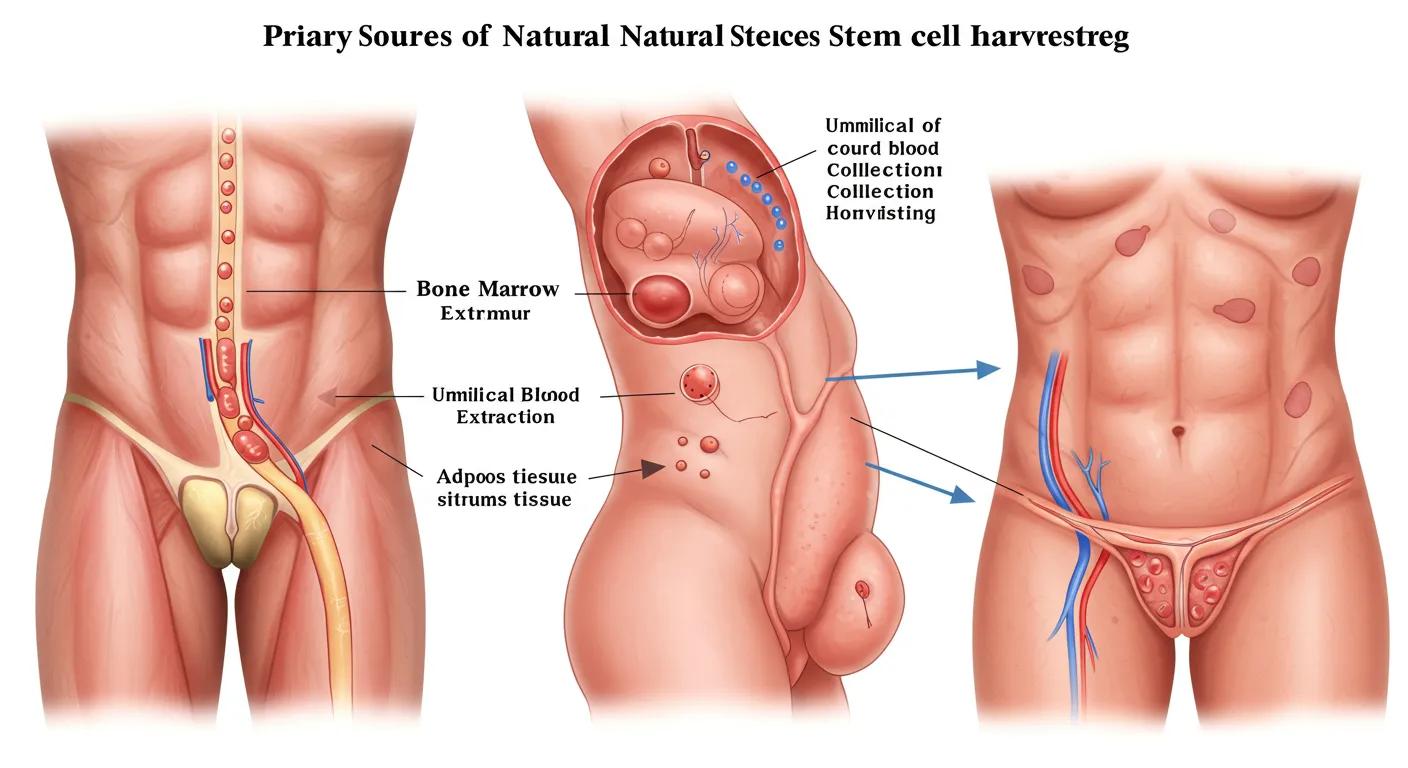
Natural stem cells are undifferentiated cells obtained directly from an organism’s tissues, offering innate repair potential due to their native microenvironment and lineage bias. Harvested from bone marrow, umbilical cord blood, and adipose tissue, these cells provide immediate therapeutic benefit in hematopoietic restoration and tissue regeneration. For example, bone marrow–derived hematopoietic stem cells reconstitute blood cell lineages in leukemia patients.
Natural stem cell sources vary by tissue, potency, and harvest method:
- Bone marrow delivers hematopoietic stem cells through aspiration that restore blood-forming capacity.
- Umbilical cord blood yields pluripotential cells with reduced graft-versus-host risk in pediatric transplants.
- Adipose tissue supplies mesenchymal stromal cells via liposuction for orthopedic and immunomodulatory applications.
Each source’s unique biological niche influences proliferation, differentiation bias, and immunogenicity, laying the groundwork for autologous or donor-derived therapies that follow.
Which Tissues Provide Natural Stem Cells?
Hematopoietic and mesenchymal lineages originate from distinct anatomical sites: bone marrow cavities, placental and cord vessels, and subcutaneous fat. Bone marrow provides HSCs that differentiate into all blood cells, while adipose tissue yields MSCs capable of forming bone, cartilage, and fat cells. Cord blood offers a mixed population with higher proliferative potential and lower immune rejection risk, bridging pediatric to adult therapy.
What Are Autologous and Allogeneic Stem Cells?
Autologous stem cells derive from the patient’s own tissues, ensuring genetic match and eliminating rejection risk. Allogeneic stem cells come from donors, enabling off-the-shelf treatments but requiring HLA matching and immunosuppression to prevent graft-versus-host disease. Autologous therapies minimize immune barriers, whereas allogeneic approaches optimize availability and rapid deployment.
How Are Natural Stem Cells Used in Therapy?
Natural stem cells serve primarily in transplantation for hematologic disorders and regenerative medicine. Autologous bone marrow transplants treat blood cancers by reinfusing a patient’s own HSCs after high-dose chemotherapy. Cord blood transplants leverage immature immune profiles for pediatric leukemia. Adipose-derived MSCs are injected into joints to reduce inflammation and support cartilage repair.
What Are the Benefits of Autologous Stem Cell Therapy?
Autologous treatments enhance safety through perfect genetic compatibility, removing the need for lifelong immunosuppression and reducing infection and rejection rates. They facilitate outpatient procedures with lower regulatory barriers, as cells return to the donor without cross-species or third-party concerns, improving patient outcomes and recovery times.
How Are Lab-Grown Stem Cells Created and What Types Exist?

Lab-grown stem cells are generated through in vitro culture or reprogramming techniques that expand or reset cellular potency, enabling unlimited supply and standardized quality control. Induced pluripotent stem cells (iPSCs) are reprogrammed from adult somatic cells using transcription factors, providing patient-specific pluripotent lines for personalized therapy. Embryonic stem cells (ESCs) derive from blastocysts and offer robust differentiation but raise ethical considerations. Cultured mesenchymal stem cells (MSCs) are expanded from adult tissues under controlled conditions for immunomodulatory use. Controlled differentiation via growth factors and small molecules directs lineage commitment, while CRISPR-based gene editing refines safety and functional performance, as seen in preclinical disease-model corrections.
What Are Induced Pluripotent Stem Cells (iPSCs) and Their Applications?
iPSCs are somatic cells reverted to a pluripotent state by introducing defined factors, unlocking patient-specific disease modeling, drug screening, and autologous regenerative treatments. They have been differentiated into dopaminergic neurons for Parkinson’s disease models and retinal pigment epithelium for macular degeneration, illustrating broad therapeutic potential.
Induced Pluripotent Stem Cells: Ethical Alternatives to Embryonic Stem Cells
Direct reprogramming enables the generation of pluripotent stem-cell lines from almost any somatic tissue and mammalian species, thereby avoiding the ethical issues associated with human embryonic stem cells.
Methods for making induced pluripotent stem cells: reprogramming a la carte, S Boué, 2011
How Do Embryonic Stem Cells (ESCs) Differ in Research and Ethics?
ESCs are derived from early embryos and exhibit true pluripotency with high proliferative capacity. Their use accelerates fundamental research and potential organoid creation but raises ethical debates over embryo destruction. Regulatory frameworks differ by region, requiring careful consent and oversight.
What Role Do Cultured Mesenchymal Stem Cells (MSCs) Play in Therapy?
Cultured MSCs offer robust immunomodulatory and trophic support, making them candidates for graft-versus-host disease mitigation, osteoarthritis treatment, and wound healing. Standardized protocols expand MSCs in bioreactors, ensuring consistent potency and viability for off-the-shelf allogeneic applications.
How Is Stem Cell Differentiation Controlled in the Lab?
Differentiation is guided by applying growth factors, small molecules, and extracellular matrix cues in defined sequences, mimicking embryonic development. For example, adding BMP4 and Activin A directs pluripotent cells toward mesoderm, while Wnt modulation refines cardiac lineage commitment, enabling targeted tissue engineering.
What Advances Are Being Made in Gene Editing of Lab-Grown Stem Cells?
CRISPR-Cas9 and base editing allow precise correction of genetic defects in iPSCs before differentiation, enhancing safety and function. For instance, corrected iPSCs transplanted into disease models of sickle cell anemia restore normal hemoglobin production, demonstrating therapeutic feasibility.
What Are the Key Differences Between Natural and Lab-Grown Stem Cells?
Natural and lab-grown stem cells differ primarily in origin, immune profile, scalability, ethical considerations, cost, and clinical track record. Natural cells come with predefined lineage bias and immediate compatibility, while lab-grown cells offer unlimited supply, uniform quality, and customizable properties. Ethical debates center on embryonic sources versus reprogrammed adult cells. Cost structures vary: harvesting and banking natural cells versus manufacturing and quality control of cultured lines. Safety profiles reflect known risks of natural transplantation—graft-versus-host disease for allogeneic cells—and emerging concerns of teratoma formation in pluripotent lines. Availability hinges on donor supply versus bioreactor expansion, shaping accessibility for large-scale treatments.
How Does Immune Response Vary Between Natural and Lab-Grown Stem Cells?
Natural autologous cells bypass immune surveillance, while allogeneic cells require HLA matching and immunosuppression to prevent rejection. Lab-grown iPSCs can be derived autologously or engineered to lack HLA antigens, reducing immunogenicity but necessitating rigorous validation to avoid unintended immune activation.
Immune Regeneration After Stem Cell Transplantation: Autologous vs. Allogeneic
Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade
Enhanced immune system regeneration in humans following allogeneic or autologous hemopoietic stem cell transplantation by temporary sex steroid blockade, JS Sutherland, 2008
How Do Availability and Scalability Compare?
Natural stem cells depend on donor availability and collection logistics, limiting batch sizes for rare tissues. Lab-grown cells benefit from scalable bioreactor systems, enabling large-scale production of uniform batches suitable for broad therapeutic distribution and off-the-shelf applications.
What Are the Ethical Considerations for Each Stem Cell Type?
Harvesting adult stem cells raises few ethical issues, while ESC research involves embryo destruction, prompting regulatory restrictions. iPSCs circumvent these concerns by reprogramming adult cells without embryonic use, positioning them as ethically favorable alternatives.
How Do Cost and Accessibility Differ?
Natural cell therapies incur expenses in donor screening, harvesting, and banking but benefit from established reimbursement pathways. Lab-grown therapies incur higher R&D and manufacturing costs, variable coverage, and limited production capacity, influencing patient access and insurance approval.
What Are the Comparative Safety and Efficacy Profiles?
Natural autologous transplants show low complication rates and predictable engraftment, while allogeneic grafts carry graft-versus-host risk. Lab-grown cells offer customized properties but pose tumorigenicity and immunogenic uncertainties that require long-term monitoring in clinical trials.
How Are Stem Cell Transplantations Performed Using Natural and Lab-Grown Cells?
Stem cell transplantation uses either harvested natural cells or cultured cells prepared under Good Manufacturing Practice (GMP) conditions to replace or repair damaged tissues. Both approaches involve cell collection, conditioning regimens, infusion protocols, and post-transplant monitoring to ensure engraftment and minimize complications. Advances in cell processing, cryopreservation, and immunomodulation optimize outcomes across sources.
What Is the Process of Bone Marrow and Cord Blood Stem Cell Harvesting?
Bone marrow harvesting uses needle aspiration under anesthesia to withdraw HSCs from pelvic sites, followed by density gradient separation. Cord blood collection occurs at birth by clamping and draining the umbilical vein into collection bags, then processing to isolate stem cells for cryogenic storage.
How Are Lab-Grown Stem Cells Prepared for Transplantation?
Lab-grown cells undergo expansion in GMP-approved bioreactors, quality control assays for sterility, potency, and karyotype stability, and directed differentiation to appropriate lineages. Cells are washed, concentrated, and formulated in infusion media following rigorous release criteria before patient infusion.
What Are the Challenges of Immune Response in Allogeneic Transplants?
Allogeneic transplants risk graft-versus-host disease when donor T cells attack recipient tissues. Strategies such as T-cell depletion, HLA matching, and immunosuppressive regimens reduce incidence but increase infection risk. Emerging approaches include regulatory T-cell infusions and gene editing to knock out HLA loci.
How Do Autologous Transplants Minimize Immune Complications?
Autologous transplants eliminate graft-versus-host disease by using the patient’s own cells, requiring only supportive care rather than immunosuppression. This approach reduces opportunistic infection rates and improves long-term survival, especially in hematologic malignancies.
Immune Reconstitution Following Autologous Stem Cell Transplant
Immune reconstitution after autologous hematopoietic stem cell transplantation
Immune reconstitution after autologous hematopoietic stem cell transplantation, 2001
What Are the Current and Emerging Applications of Natural and Lab-Grown Stem Cells?
Stem cell therapies address hematologic cancers, genetic disorders, orthopedic injuries, and degenerative diseases through both natural and engineered cells. Emerging applications include cardiac repair using MSC patches, neural regeneration with iPSC-derived neurons, and organoid models for personalized drug screening. Clinical trials are investigating combined cell and gene therapies for muscular dystrophy and liver failure.
- Hematopoietic restoration in leukemia and lymphoma
- Cartilage repair in osteoarthritis and sports injuries
- Neurodegenerative disease modeling and treatment
- Organoid-based drug discovery platforms
Which Diseases Are Treated with Natural Stem Cell Therapies?
Natural HSC transplants cure blood cancers such as leukemia and lymphoma, while MSC injections alleviate symptoms of osteoarthritis and graft-versus-host disease. Cord blood units treat pediatric immunodeficiencies and hemoglobinopathies.
What Are the Latest Breakthroughs in Lab-Grown Stem Cell Therapies?
iPSC-derived retinal cells have restored vision in macular degeneration trials, and corrected iPSC-based cardiomyocytes show promising engraftment in heart failure models. Gene-edited MSCs targeting inflammatory cytokines are in Phase II studies for autoimmune conditions.
How Are Clinical Trials Shaping the Future of Stem Cell Treatments?
Ongoing trials evaluate safety, dosing, and efficacy of both natural and lab-grown cells in diverse indications, generating data on long-term engraftment, functional outcomes, and immune modulation. Adaptive trial designs accelerate translation from bench to bedside.
What Is the Role of Regenerative Medicine in Stem Cell Therapy?
Regenerative medicine integrates scaffolds, growth factors, and cell therapies to repair or replace damaged tissues. Combining natural and engineered cells within biomaterial matrices enhances structural support and functional recovery in complex injuries and chronic degenerative diseases.
What Are the Advantages and Limitations of Autologous vs Allogeneic Stem Cells?
Autologous cells guarantee compatibility and safety but require individualized harvesting and processing, delaying treatment. Allogeneic cells provide ready-to-use therapies with standardized quality but face immune barriers and ethical sourcing challenges. Balancing these factors guides patient-specific decisions.
How Does Donor Matching Affect Allogeneic Stem Cell Success?
HLA matching between donor and recipient reduces graft-versus-host disease and improves engraftment. Mismatches increase immune activation, requiring intensified immunosuppression with associated toxicity.
What Are the Risks and Benefits of Using Autologous Cells?
Autologous cells eliminate rejection and decrease infection risk, improving safety profiles. However, patient health and prior treatments may impair cell yield and function, limiting efficacy in heavily pretreated individuals.
How Do Immune Responses Impact Transplant Outcomes?
Host versus graft reactions can destroy transplanted cells, while graft-versus-host disease causes tissue damage. Immunosuppressive protocols aim to strike balance between preventing rejection and preserving immune defense against infections and malignancy recurrence.
What Are the Practical Considerations for Patients Choosing Between These Options?
Patients must weigh urgency of treatment, donor availability, cost, potential delays, and risk tolerance. Autologous therapy suits elective regenerative applications, while allogeneic cells serve acute conditions requiring immediate intervention.
What Is the Future Outlook for Natural and Lab-Grown Stem Cell Technologies?
Advancements in gene editing, bioreactor design, personalized reprogramming, and automated manufacturing are poised to transform regenerative treatments. Integration of CRISPR-edited iPSCs, 3D bioprinted tissues, and patient-matched robotics heralds an era of bespoke organ replacement and disease reversal.
How Will Gene Editing Improve Lab-Grown Stem Cell Therapies?
Precise correction of disease-causing mutations in iPSCs using CRISPR and base editors enables autologous cell therapies free of inherited defects. Edited cells differentiate into healthy tissue, reducing relapse and improving functional outcomes.
What Emerging Technologies Are Enhancing Stem Cell Differentiation Control?
Microfluidic bioreactors, three-dimensional organoid cultures, and synthetic extracellular matrices provide spatial and temporal cues that yield uniform, mature cell populations. These platforms enhance reproducibility and scale-up for clinical deployment.
How Is Personalized Medicine Shaping Stem Cell Treatments?
Patient-derived iPSCs and customized biomaterial scaffolds enable truly personalized grafts tailored to individual genetic profiles, disease phenotypes, and immune landscapes. Combined omics and artificial intelligence guide optimal cell sourcing, differentiation protocols, and dosing regimens.
What Market Trends Are Driving Growth in Stem Cell Research and Therapy?
Rising investments in regenerative medicine, favorable regulatory pathways for advanced therapies, and growing chronic disease burdens fuel market expansion projected at a double-digit CAGR through 2034. Strategic partnerships between biotech firms, academic centers, and healthcare systems accelerate translation of stem cell innovations.
Natural and lab-grown stem cells have distinct origins, advantages, and challenges that complement each other in modern medicine. Autonomous harvesting and donor banking provide proven routes for hematologic and regenerative therapies, while engineered pluripotent lines unlock personalized, scalable treatments. Ongoing research in gene editing, differentiation platforms, and organoid engineering promises to converge these approaches into seamless regenerative solutions that address unmet clinical needs and redefine therapeutic possibilities.
Authored By:
Dr. Charles Pereyra
Regenerative Health Expert
Founding Physician at Springs Rejuvenation